Another Advance for the Healthcare Revolution: SNGULAR Trials Unlocks Decentralised Clinical Trials
April 19, 2024
In a world where technology is allowing us to reach limits we thought impossible just a few years ago, medicine and healthcare are ready for the revolution, and so are the patients. SNGULAR health has created SNGULAR Trials as part of this technological revolution. SNGULAR Trials is a platform that allows clinical trials to be conducted remotely by connecting patients to medical staff without trips to the hospital or clinic. Using this platform, medical staff can define the clinical trial, follow-up on participants’ progress, provide guidance to the participants, and analyze the results.
Background
Clinical trials are a type of research that studies new tests and treatments and characterises and evaluates their effects on human health. A clinical trial can evaluate new drugs, medical devices, vaccines, surgical and other medical treatments, and/or medical procedures. These tests are carefully designed and reviewed before they are approved. People of all ages can participate as volunteers.
These are the four phases of biomedical clinical trials:
- Phase I studies usually test new drugs for the first time in a small group of people to evaluate a safe dosage range and identify side effects.
- Phase II studies test treatments that have been found to be safe in phase I but now need a larger group of human subjects to monitor for any adverse effects.
- Phase III studies are conducted on larger populations and in different regions and countries and are often the step right before a new treatment is approved.
- Phase IV studies take place after the trial is approved and when there is a need for further testing in a wide population over a longer timeframe.
Challenges
However, the challenges faced by these clinical trials grow as fast as the need for them. Hundreds of clinical professionals representing pharma, CROs, sites, and vendors were asked about the biggest challenges they are facing. The graph below shows their most common responses:
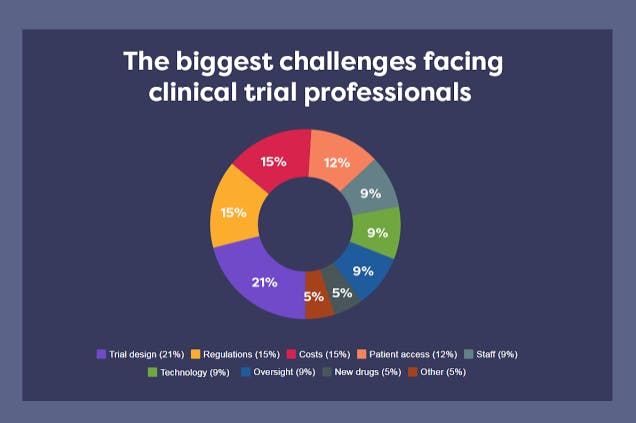 Figure 1: Pie chart with the results of the interviews to clinical trials professionals. From InformaConnect article.
Figure 1: Pie chart with the results of the interviews to clinical trials professionals. From InformaConnect article.
The data in this graph pinpoints some key challenges that can be addressed through technology. These problems are the complexity of trial design (21 %), the costs (15%), and patient access (12%).
Solution
SNGULAR Trials addresses these three problems of clinical trials by streamlining physicians’ work, optimizing systems, improving patient outcomes, reducing human error, connecting patients to staff, and lowering costs through engaging web and mobile experiences. SNGULAR Trials offers a more efficient way of carrying out these clinical trials by making them more digital, decentralized, and flexible. The platform also provides physicians the most information and the most accurate data for each study while it keeps the participants informed and engaged.
SNGULAR Trials reduces healthcare professionals’ workload and staff as well as overall logistic complexity and costs. Participants can communicate with the hospital and provide the required information for the clinical trial. Remote follow-up leaves the hospital visits only for those moments in which they are actually required and reduced. In addition, the platform reduces the impact on the participants’ day-to-day life because they are no longer required to travel to specific locations regularly. This benefit can have a huge impact on patient access limitations, the easier it is to participate, the easier it is to get patients involved in the studies, and this increased access will make the trials more complete, effective and of a better quality.
Process
Through the SNGULAR Trials application, doctors, nurses, and healthcare professionals have access to the trials by logging onto the platform. This platform allows them to: (1) create a new clinical trial and configure it, including the subjects in each trial phase; (2) gain access to external labs or other hospital areas; and (3) be in touch with the participants (via texts and video calls).
Participants follow the step-by-step clear instructions in the app such as when to take a pill, eat, drink, or engage in a physical activity, and report how they feel or any symptoms or changes they might experience throughout the course of the study. They can also contact the hospital via text or video call and, in case of emergency, they can press the “Need Help” button in the app, which will result in immediate attention.
For certain studies, participants might also be asked to use wearables or testing devices to measure specific parameters (heart rate, blood pressure, blood sugar, etc.) that professionals need to record. Data can be obtained from any device with Bluetooth capabilities, and the platform is compatible with most healthcare wearable devices currently on the market. The platform can also be integrated with VR glasses and document signature applications. Measurements registered into the device can be viewed by both the participant (from the mobile app) and the doctor or nurse (from the web platform). Thus, SNGULAR Trials allows institutions interested in conducting clinical trials to monitor participants’ vital signs in real time and analyse the parameters using big data technologies.
In addition, all trial and patient data as well as lab and test results are put together and combined in a safe, consistent manner complying with the Consumer Privacy Act.
Conclusion
SNGULAR Trials helps doctors and nurses accelerate and perform data-driven decisions and processes to detect clinical patterns. It also unlocks easy, cost-effective clinical trial follow-up and logistics while patients remain safe and monitored with minimal disruption in their lives. Finally, it advances the healthcare revolution by fostering participant engagement in clinical trials and, ultimately, saving lives.